Description
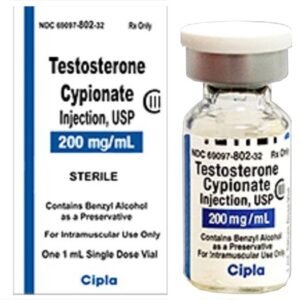
Testosterone Enanthate Injection is a compounded intramuscular androgen formulation prepared in sterile grapeseed oil at a concentration of 200 mg/mL and dispensed in multi-dose 5 mL vials for prescription-only use. It is indicated for testosterone-replacement therapy in adult males with clinically confirmed hypogonadism and for select female patients with inoperable metastatic breast cancer; all other uses remain off-label and must be justified by individualized clinical judgment. Because it is compounded under section 503A, the preparation is not evaluated by the U.S. Food and Drug Administration for safety, efficacy, or bioequivalence, and practitioners must therefore rely on primary literature, clinical guidelines, and therapeutic monitoring to mitigate risk.
Pharmacologically, the depot nature of the enanthate ester confers a prolonged release profile relative to unesterified testosterone, allowing dosing intervals of one to four weeks while still producing considerable peak-to-trough variability. Individualized titration is essential to achieve serum trough levels in the mid-normal physiologic range, thereby minimizing adverse effects such as erythrocytosis, gynecomastia due to aromatization, or suppression of endogenous spermatogenesis.


Reviews
There are no reviews yet.