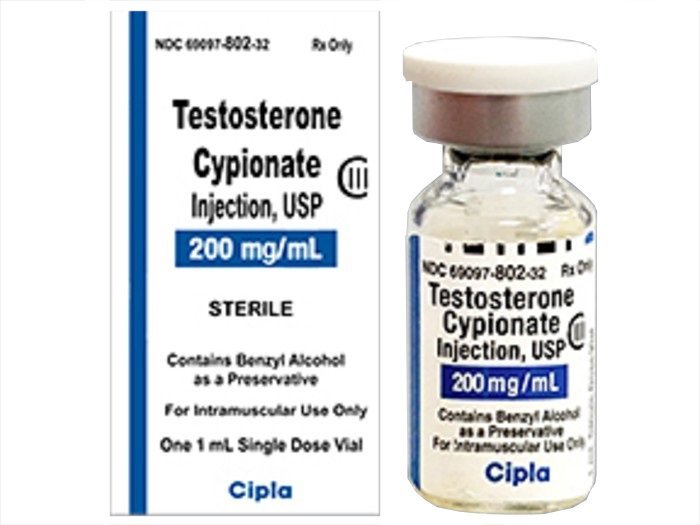
Description
| McKesson # | 1134364 |
|---|---|
| Manufacturer # | 69097080232 |
| Manufacturer | Cipla USA |
| Country of Origin | Unknown |
| Alternate Manufacturer Number | 3937802 |
| Application | Androgen |
| Buy American Act (BAA) Compliant | No |
| Container Type | Single-Dose Vial |
| Dosage Form | Injection |
| Drug Schedule | CIII |
| Generic Drug Code | 10194 |
| Generic Drug Name | Testosterone Cypionate |
| NDC Number | 69097080232 |
| Strength | 200 mg / mL |
| Trade Agreement Act (TAA) Compliant | No |
| Type | Intramuscular |
| UNSPSC Code | 51182069 |
| Volume | 1 mL |

Reviews
There are no reviews yet.